Efficacy of Cranial Electrotherapy Stimulation on Anxiety and Sleep Qualities Among Elderly Patients with Chronic Sleep Disorders.
Ren, Y., Zhu, Y., Yang, J., & Ding, F. Efficacy of Cranial Electrotherapy Stimulation on Anxiety and Sleep Qualities Among Elderly Patients with Chronic Sleep Disorders. Chinese Journal Modern Nursing. 2019; 25(24).
Funding Source, Location of Study or Author’s Affiliation
Geriatric Department and Nursing Department, the First Affiliated Hospital of Chongqing Medical University, Chongqing, China.
Device
Alpha-Stim® SCS
Key Variables
Anxiety & Sleep.
Objective
To explore the effects of cranial electrotherapy stimulation (CES) on anxiety and sleep quality among elderly patients with chronic sleep disorders.
Design
A randomized controlled trial of 120 elderly patients with chronic sleep disorders was randomly divided into a control and treatment group, with 60 cases in each group. The control group MED received treatment-as-usual (TAU) that involved medication, either clonazepam or ramelteon. The treatment group CES received a course of CES treatment constituting once daily, 20 minutes session, over 12 weeks. The sleep quality and anxiety of patients in the two groups were evaluated using the Pittsburgh Sleep Quality Index (PSQI) and the Hamilton Anxiety Rating (HAM-A) scale before treatment and after a course of treatment.
Primary Outcome Measure
- Sleep quality and quantity as measured by the PSQI, a 19-item scale to measure sleep quality and disturbance over two weeks. The PSQI includes 7 factors and 19 self-evaluation items, the scores range for each factor from 0-3. Higher PSQI scores indicate poorer sleep quality.
- Anxiety as measured by the HAM-A scale before treatment and after a course of treatment. The HAM-A total score (i) < 7 indicates “No anxiety”, (ii) 7-13 “Possible anxiety”, (iii) 14-20 “Definite anxiety”, (iv) 21-28 “Definite and obvious anxiety”, and (v) ≥ 29 “Possibly severe anxiety”.
Secondary Outcome Measure
None reported.
Key Inclusion Criteria
- Sleep disturbance
- meet the diagnostic criteria insomnia specified in the Chinese Classification of Mental Disorders (CCMD-3).
- latency to persistent sleep (LPS) is prolonged (>30 minutes).
- actual sleep time is reduced (<6 hours every night).
- a disease course of more than 6 months.
- Aged ≥ 60 years.
Key Exclusion Criteria
- Significant or severe organ dysfunction, malignant tumors, other end-stage diseases.
- Severe cognitive disorders.
- Sleep apnea syndrome.
- Sleep disorders caused by severe mental disorders.
- Receiving long-term drug or other treatments.
- Not taking an antidepressant, anxiolytics, sleep medication, or other psychotropic drugs within the 2 weeks before the start of the study.
Protocol Summary
A group of 120 elderly patients with chronic sleep disorders was randomly divided into a control group (n=60 MED) receiving standard medical care that involved medication and an experimental treatment group (n=60 CES) that received a course of CES treatment constituting a once-daily, 20 minutes session, over 12 weeks. The sleep quality and anxiety of patients in two groups were evaluated at baseline and post-treatment using the PSQI and HAM-A scale.
Patients in both groups received routine nursing care and regular telephone follow-up with information aimed at promoting general health, instructions on drug use, medication reminders, Q&A opportunities, psychological support, and reminders to return to outpatient visits.
In the MED group, patients took sleep medication as prescribed by their doctor – typically 2mg qd hs of Clonazepam (specification: 0.5mg per tablet) or of 4mg qd hs Ramelteon (specification 8mg per tablet) every day. Patients in the experimental CES group received CES treatment for 20 minutes before falling asleep every day for 12 weeks.
Before the intervention, informed consent was obtained from the patient; inclusion criteria were reviewed; patient’s clinical data, including medical history, was collected, a physical assessment was conducted, and vital signs were collected. The medical team also provided an auxiliary examination and reviewed past or concomitant medications.
Device Application Protocol
The CES group received CES treatment using the Alpha-Stim® SCS. The patient’s earlobes were thoroughly cleaned with mild soapy water, dried, and the electrodes were attached to the earlobes near the jawbone. The current intensity was adjusted from the minimum value and gradually increased until the patient felt a comfortable mild tingling sensation.
All patients received CES treatment for 20 minutes before falling asleep every day for 12 weeks. During treatment, patients were advised to relax, and engage in activities associated with minimal movements, such as reading a book or newspaper, but were asked to not talk with others during treatment.
Statistical Analysis Plan
SPSS 23.0 statistical software was used for data analysis. The measurement data were expressed as mean ± standard deviation (SD), and the enumeration data were expressed with frequency and percentage (%). Significance testing using t-test and χ2 test were carried out for independent samples, and p<.05 level equated to a statistically significant difference.
Results
Subjects
In total 120 patients were enrolled in the study, with 60 randomly allocated into the MED and CES. Subjects were withdrawn from the study if they showed (1) adverse reaction(s) during the intervention, (2) failure to complete the study for any reason. Consequently, a total of 52 patients in the MED group and 55 in the CES group completed the study. Baseline comparison measures showed no statistically significant differences between the patients in each group (p>.05) shown in the table below.
| GROUP | Cases | Males | Female | Age Years (mean/SD) | Disease Course Months (mean/SD) |
| MED | 52 | 28 | 24 | 65.2 + 4.2 | 24.5 + 10.2 |
| CES | 55 | 30 | 25 | 66.8 + 5.9 | 26.1 + 13.0 |
| X2/t value | 0.005 | -1.623 | – 0.71 | ||
| p-value | 0.942 | 0.108 | 0.479 |
Comparison of general data in elderly patients with chronic sleep disorders between both groups.
Data Analysis
The results showed that the therapeutic effect of CES was better than that of traditional drug therapy (p<.001).
After the intervention, the total PSQI scores of the treatment group (CES) were lower (7.08±0.98) than those of the control group (MED) (8.97±1.38), indicating greater improvement in sleep quality, and the difference was statistically significant (t=8.127, p<.001).
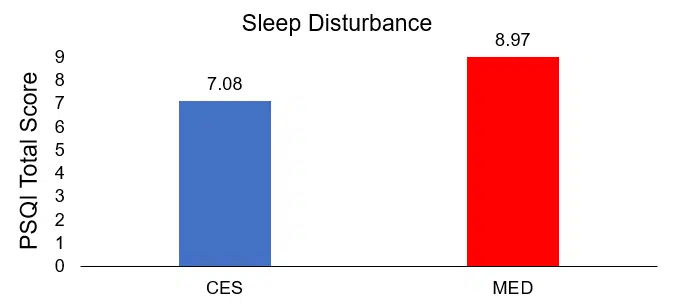
Comparison of posttreatment differences in sleep disturbance
Moreover, according to HAM-A scores, CES treatment was better than the MED group at improving sensory system symptoms, gastrointestinal tract symptoms, genitourinary system symptoms, and autonomic nervous system symptoms, etc. (p<.001). The change in HAM-A scores suggested that CES improves sleep and anxiety; in addition to improving the sensory system, gastrointestinal tract, genitourinary system, and autonomic nervous system symptoms (p<.001).
| GROUP | Cases | Total Score | Anxiety | Nervousness | Fear | Insomnia | Cognitive Disorder | Depression | Somatic Anxiety |
| CES | 52 | 8.12+3.11 | 1.02+0.24 | 0.89+0.07 | 0.42+0.09 | 1.08+0.17 | 1.42+0.21 | 0.87+0.11 | 0.71+0.07 |
| MED | 55 | 13.12+5.12 | 1.56+0.19 | 1.01+0.17 | 0.58+0.07 | 1.98+0.41 | 1.58+0.19 | 1.11+0.09 | 0.78+0.09 |
| t value | -6.063 | -12.940 | -4.726 | -10.296 | -14.681 | -4.125 | -12.381 | 4.504 | |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| GROUP | Cases | Sensory System | Cardiovascular System | Respiratory System | Gastrointestinal System | Genitourinary System | Autonomic Nervous System | Performance When Talking With Others | ||||
| CES | 52 | 0.58±0.47 | 1.37±0.11 | 0.34±0.11 | 0.82±0.18 | 0.47±0.13 | 0.47±0.18 | 0.21±0.07 | ||||
| MED | 55 | 1.74±0.18 | 1.38±0.07 | 0.58±0.21 | 2.37±0.29 | 0.85±0.07 | 1.57±0.17 | 0.44±0.15 | ||||
| t value | -17.030 | -0.564 | 7.462 | -32.998 | -18.964 | -32.510 | -10.069 | |||||
| p value | <0.001 | 0.574 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||
Comparison of anxiety (HAM-A) scores after treatment between the control (MED) and experimental group (CES).
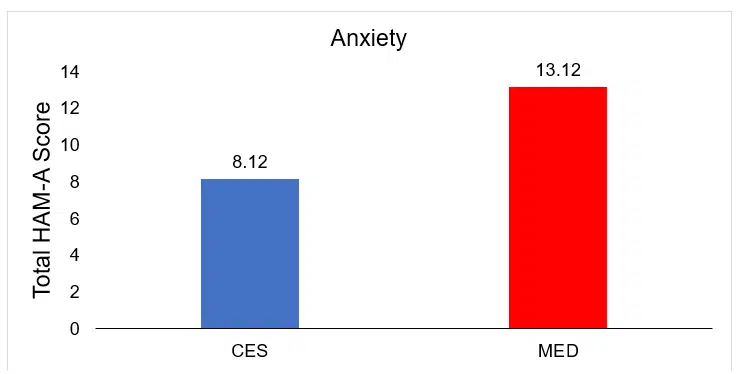
Comparison of posttreatment differences in anxiety
Conclusion
The authors conclude that, compared with traditional medication treatments, CES more effectively improves sleep quality and reduces anxiety in elderly patients with chronic sleep issues, without adverse reactions. The results of the HAM-A demonstrate CES also improved the physiological symptoms of anxiety more effectively than medication.
Limitations
Only the post treatment scores on the PSQI and HAM-A are provided, so the progress throughout the 12-week treatment period cannot be determined. Additionally, participants in the CES condition treated before going to bed, despite the Instructions for Use (IFU) for Alpha-Stim advising against treating within 3-4 hours of going to sleep.
Study Quality: FAIR
