Comparative Observation between Single Use of Fluoxetine & Its Combination with CES
Luo, H-D, Luo, H-L., Hong, L-F Comparative Observation between Single Use of Fluoxetine and Its Combination with CES. Chin J Mod Drug Appl. 2015; 9(7).
Funding Source, Location of Study, or Author’s Affiliation
Meizhou City People’s Hospital, Meizhou, China
Device
Alpha-Stim®
Key Variables
Depression
Objective
Compare the clinical effect of fluoxetine combined with Cranial Electrotherapy Stimulation (CES) in the treatment of depression.
Design
A total of 90 patients diagnosed with depression were randomly divided into two treatment groups (1) experimental (fluoxetine combined with CES), and (2) control group (fluoxetine only). Each group contained 45 cases. After four weeks of treatment and using the Hamilton Depression Rating Scale (HAM-D) and Treatment Emergent Symptom Scale (TESS) the impact of the treatment conditions were evaluated for efficacy, speed of response, any adverse reactions.
Primary Outcome Measure
Measures were taken at baseline, two weeks, and four weeks post-treatment
- Change is depression scores as measured by HAM-D
- Assessment of side effect profile and treatment response as measured by TESS
Secondary Outcome Measure
Routine assessment at baseline, end of weeks: 1, 2, 3, 4, and post-treatment of
- Blood
- Urine
- Stool
- Liver function
- Electrocardiogram (ECG)
- Electroencephalogram (EEG)
Key Inclusion Criteria
- Depression diagnosis as specified in Chinese Classification and Diagnostic Criteria of Mental Disorders, Edition 3 (CCMD-3)
- A total score of 17 items on the HAMD ≥18
- Discontinuation of all medications
- Willingness to be treated with 20mg of fluoxetine daily, increasing to 30mg daily
- No additional psychiatric disorders
- Absence of significant
- Cognitive or intellectual impairment
- Communication or comprehension difficulties inclusive of aphasia
Key Exclusion Criteria
- Severe heart, liver, or kidney disease
- Epilepsy
- Glaucoma
Protocol Summary
Discontinuation of all medications with a wash-out period of 1 week, and random allocation into two groups of 45.
- Control group received Treatment As Usual (TAU) which consisted of fluoxetine 20mg daily after breakfast, increased to 30mg/d within two weeks until the end of four weeks.
- Experimental group received fluoxetine (TAU) in addition to CES, twice daily for 30 minutes for three weeks, and then once daily every two days until the end of week 4.
During the treatment, patients could also take Inderal, Vitamin B6, or a small dose of benzodiazepines based on their conditions. Routine blood, urine, stool, and liver function tests were performed, in addition to ECG and EEG before treatment and at the end of weeks 1, 2, 3, and 4 of treatment.
Device Application Protocol
Treatment time for CES using Alpha-Stim was 30 minutes two times per day for the first three weeks and one treatment every two days for the final week. The course of treatment was four weeks.
Statistical Analysis Plan
Statistical analysis using SPSS18.0 statistical software, with measurement data expressed with mean ± standard deviation (SD) and tested by t-test. Data were expressed with rate (%) and were tested by χ2 test. A significance level of p<0.05 indicated a statistically significant difference.
Change in HAM-D was used to evaluate the degree of depression and TESS to calculate any side effects at baseline, end of two and four weeks. The clinical effect of fluoxetine and fluoxetine + CES was categorized into four levels from highly effective to not effective.
The treatment response as measured by the TESS scale was arranged as (1) ≥75% indicating clinical recovery, (20) ≥50% indicating significant improvement, (3) ≥25% indicating improvement, and (4) <25% indicating no effect. A reduction of HAM-D scores from baseline was indicative of efficacy and the final HAM-D score was categorized as (1) ≤7 indicating no depression, (2) 8-17 indicating mild depression, (3) 18-23 indicating moderate depression, and (4) ≥24 indicating severe depression.
Results
Subjects
Total of 90 patients in two groups of 45 patients. The TAU+CES group included 23 males and 22 females with a mean (SD) age of 31.3±5.6 years and a mean (SD) course of disease of 3.56±3.3 months. In comparison, the TAU group included 21 males and 24 females with a mean (SD) age of 32.2±4.4 years and a mean (SD) course of disease of 3.21±4.2 months. The two groups were comparable as no significant differences at baseline were reported (p>0.05).
Data Analysis
After two weeks of treatment, the TAU+CES condition showed mean (SD) HAM-D scores of 15.4±3.56 and 19.5±3.14 for the TAU. The reduction in scores was a statistically significant difference between the two groups at the end of two weeks of treatment (p<0.05) in favor of TAU+CES. At week 4 post-treatment the mean (SD) HAM-D score in the TAU+CES group was 10.3±3.23 and 10.2±3.15 in the TAU condition. No statistically significant difference between the two conditions were observed at the end of four weeks of treatment. The TAU+CES mean (SD) HAM-D score at baseline was 25.3±4.10 and 26.4±3.52 in the TAU group were not significantly different. Both conditions showed significant reductions in HAM-D scores from baseline to end of treatment (p<0.05).
No significant differences were reported in the speed of response as measured by TESS scores between the two groups from baseline to end of treatment.
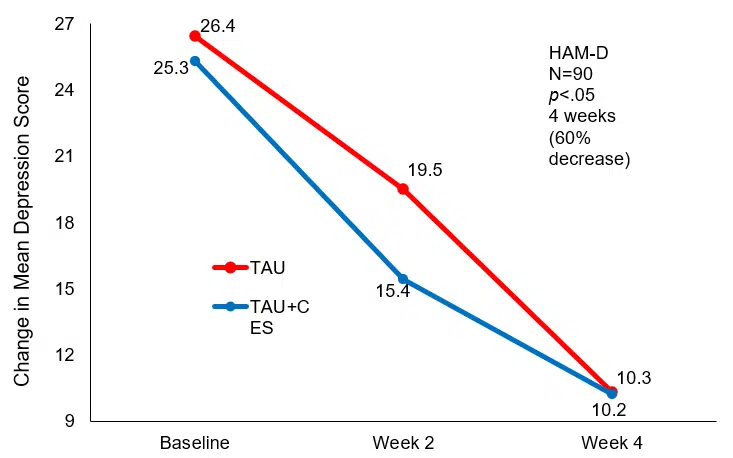
Mean Change in HAM-D score at baseline and 4 weeks post-treatment.
Conclusion
The authors reported that fluoxetine combined with CES for the treatment of depression resulted in a faster treatment response and yielded a superior therapeutic effect. The authors also concluded and recommended using CES as an augmentation strategy in the pharmacological treatment of depression. Summarizing that CES + fluoxetine reduces the duration of depression symptoms, improves treatment compliance, and limits the adverse effects of depression to patients and their families. No adverse effects were reported by combining fluoxetine with CES that were not expected by single-use fluoxetine.
Limitations
While this study was a randomized controlled trial, it was not double-blind. Therefore, both participants and investigators were aware of assigned intervention category. Additionally, the authors do not mention their procedures for missing data. According to the Cochrane risk-of-bias tool, each of these conditions can introduce unintended bias into a study.
Study Quality: FAIR
