Cranial electrotherapy stimulation affects mood state but not levels of peripheral neurotrophic factors or hypothalamic-pituitary-adrenal axis regulation
Roh, HT. & So, W-Y. Cranial electrotherapy stimulation affects mood state but not levels of peripheral neurotrophic factors or hypothalamic-pituitary-adrenal axis regulation. Technology and health care: Official Journal of the European Society for Engineering and Medicine. 2017; 25(3):403–412.
Funding Source, Location of Study, or Author’s Affiliation
Sports and Health Care Major, College of Humanities and Arts, Korea National University of Transportation, Chungbuk, Korea.
Device
Alpha-Stim® 100
Key Variables
Changes in neurotrophic factors and mood.
Objective
The present study aimed to evaluate changes in the hypothalamic-pituitary-adrenal (HPA) axis response and levels of neurotrophic factors, blood cortisol, adrenocorticotropic hormone (ACTH), neurogenesis, and neuroplasticity; as well as changes in mood state, in patients undergoing Cranial Electrotherapy Stimulation (CES) therapy.
Design
A randomized controlled trial (RCT) designed to assess changes in the HPA axis response and levels of neurotrophic factors, and changes in mood states following a period of eight weeks of treatment in a Sham comparator and Active CES group. Blood samples were collected before and following treatment and changes in mood state in both groups.
Primary Outcome Measure
Baseline and eight-week post-treatment levels of
- Cortisol
- Adrenocorticotropic Hormone (ACTH)
- Brain-Derived Neurotrophic Factor (BDNF)
- Nerve Growth Factor (NGF)
Secondary Outcome Measure
Changes in mood state were also examined at the time of blood collection using the Korean version of Profile of Mood States (KPOMS).
Key Inclusion Criteria
- Body mass index (BMI) under 30kg/m2
Key Exclusion Criteria
- Significant history of medical problems, drug use, or mental illness
- Chronic diseases such as hypertension or diabetes
- History of smoking
- Participation in a regular exercise program in the last six months
Protocol Summary
Participants were randomly assigned to either a Sham CES group (n=25) or an Active CES group (n=25). Randomization of each group was conducted using a simple online randomization algorithm (Random.org). Anthropometric measure, blood, hormone, and neurotrophic factors were measured at baseline and eight weeks post-treatment.
- Anthropometric measurements: Height, weight, and body composition were measured. Height was measured using a stadiometer, while weight and body composition were measured using an Inbody720 device that utilizes an eight-polar bio-impedance analysis method.
- Blood sampling & analyses: 8 ml blood samples were collected from cardinal veins in the forehand of each participant using a 22-gauge needle, serum separator tube, and ethylenediamine tetra-acetic acid tube. Collected blood was centrifuged at 3000 rpm for 15 minutes to analyze blood levels of cortisol, ACTH, BDNF, and nerve growth factor NGF. Samples were refrigerated at −80◦C until further analysis.
- Levels of HPA axis regulation-related hormones: Plasma cortisol and ACTH levels were analyzed via radioimmunoassay (RIA) and immunoradiometric assay (IRMA) using a Cortisol Coat-A-Count® Kit and an ACTH IRMA (CT) Kit respectively.
- Peripheral neurotrophic factor levels: Serum BDNF and NGF levels were analyzed via sandwich enzyme-linked immunosorbent assay. A Human BDNF ELISA Kit was used to analyze levels of BDNF, while an NGF sandwich ELISA Kit was used to analyze NGF levels. Levels were quantified at 450 nm absorbance using a microplate reader.
- Mood state: Changes in mood state were assessed using the Korean version of the Profile of Mood States (KPOMS), adapted from the original version developed by McNair et al. (1992). The questionnaire consists of 65 items scored along a five-point Likert scale and is divided into six sub-domains: Tension-Anxiety, Depression-Dejection, Anger-Hostility, Vigor-Activity, Fatigue-Inertia, and Confusion-Bewilderment.
Device Application Protocol
CES treatment using Alpha-Stim® 100 was conducted in 20-minute sessions, three times per week for eight weeks. Clip electrodes were attached to both earlobes of patients in the Active CES group, who received treatment with a current of 100 μA at a frequency of 0.5 Hz, according to manufacturer instructions and following the methods utilized in advanced studies by Kirsch and Nichols (2013), and Anderson et al. (2014). The same procedure was followed for the Sham CES group, though the power of device remained off.
Statistical Analysis Plan
Data are expressed as means and standard deviations (SD), and were analyzed using SPSS version 21.0. Two-way repeated-measures analyses of variance (ANOVA) were conducted to assess time and group differences in each variable. Both independent and dependent t-tests were conducted to examine significant interactions. The level of significance was set at a value of 0.05.
Results
Subjects
Fifty (50) healthy postmenopausal women were randomly assigned to either a Sham CES group (n=25) or an Active CES group (n=25).
Data Analysis
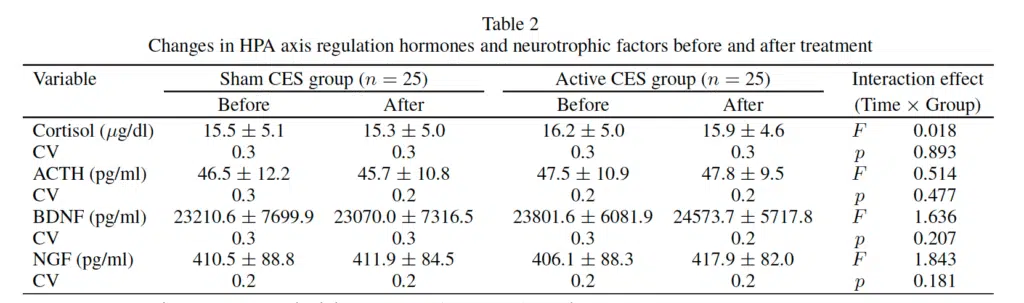
Changes in hormones associated with HPA axis regulation and neurotrophic factors following CES treatment revealed no significant differences to levels of plasma cortisol, plasma ACTH, serum BDNF, and serum NGF, or interaction between time and group following CES treatment (p>0.05).
CES (Cranial Electrotherapy Stimulation); CV (Coefficient of Variation); ACTH (Adrenocorticotropic Hormone); BDNF (Brain-Derived Neurotrophic Factor); NGF (Nerve Growth Factor).
Changes in HPA axis regulation hormones and neurotrophic factors before and after treatment.
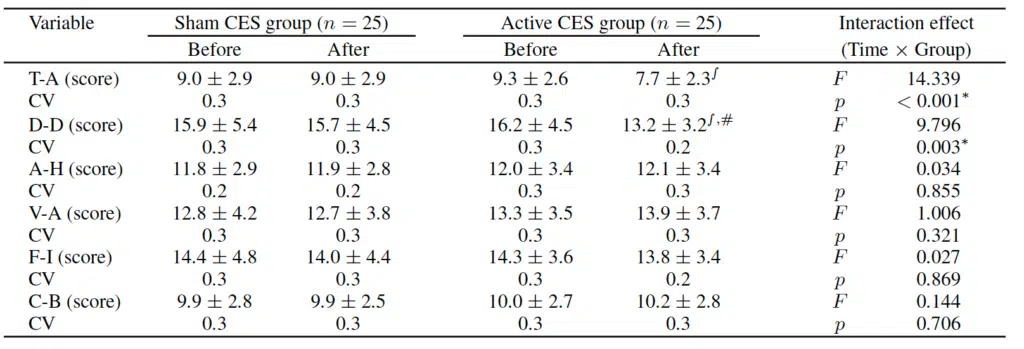
Two-way repeated-measures ANOVA for Tension-Anxiety, Depression-Dejection, Anger-Hostility, Vigor-Activity, Fatigue-Inertia, and Confusion-Bewilderment, and interaction between time and group revealed significant differences in Tension-Anxiety (F=14.339, p<0.001) and Depression-Dejection scores (F=9.796, p<0.003) following CES treatment.
CES (Cranial Electrotherapy Stimulation); CV (Coefficient of Variation); T-A (Tension-Anxiety); D-D (Depression-Dejection); A-H (Anger-Hostility); V-A (Vigor-Activity); F-I (Fatigue-Inertia); C-B (Confusion-Bewilderment) ∫ p<0.05 vs Before; #p<0.05 vs Sham CES group; ∗p<0.05 vs Before; #p<0.05 vs Sham CES group.
Changes in mood state before and after treatment.
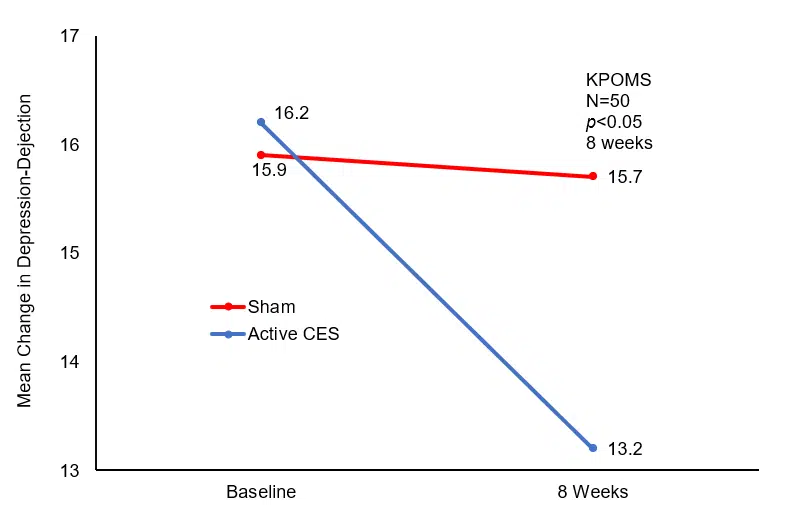
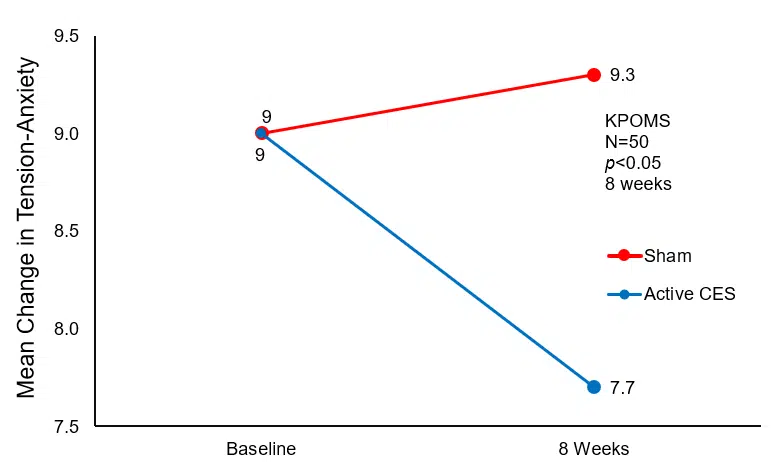
Post-hoc analysis revealed no significant differences before and after treatment in the Sham CES group, while significant decreases in Tension-Anxiety and Depression-Dejection scores (p<0.05) were observed in the Active CES group. Furthermore, Depression-Dejection scores were significantly lower in the Active CES group than in the Sham CES group (p<0.05) following treatment. However, there were no significant differences in Anger-Hostility, Vigor-Activity, Fatigue-Inertia, and Confusion-Bewilderment scores (p>0.05).
Mean Change in Depression-Dejection Baseline to Post-Treatment.
Mean Change in Tension-Anxiety Baseline to Post-Treatment
Conclusion
No significant differences in cortisol, ACTH, BDNF, or NGF were observed between the two participant groups (p>0.05) following the treatment period. However, those in the Active CES group exhibited significantly decreased Tension-Anxiety and Depression-Dejection scores on the POMS relative to pre-treatment scores (p<0.05). Furthermore, Depression-Dejection scores following treatment were significantly lower in the Active CES group than in the Sham CES group (p<0.05). No significant differences were observed in any other POMS scores such as Anger-Hostility, Vigor-Activity, Fatigue-Inertia, and Confusion-Bewilderment (p>0.05).
The authors concluded that their results suggest that eight weeks of CES treatment does not induce changes in blood levels of neurotrophic factors or HPA-axis-related hormones, though such treatment may be effective in treating symptoms of anxiety and depression.
The absence of significant changes in plasma cortisol or ACTH levels are at odds with patients reports of significant improvement in mood states related to anxiety and depression. Further studies should utilize neuroimaging methods such as functional magnetic resonance imaging (fMRI), single-photon emission computed tomography (SPECT), and positron emission tomography (PET) should be conducted in order to examine the effect of CES treatment on brain activity.
Limitations
The CES protocol utilized in this study is not consistent with other studies or with clinical instructions for use regarding current intensity and duration of treatment. At 100 uA, 60 minutes of treatment is recommended. Additionally, it is likely that a longer-term study is needed to demonstrate chemical changes from Alpha-Stim CES treatment. Additionally, the authors do not discuss a plan for missing data in their statistical analysis.
Study Quality: FAIR