Cumulative Response from Cranial Electrotherapy Stimulation (CES) for Chronic Pain
Holubec, JT. Cumulative response from cranial electrotherapy stimulation (CES) for chronic pain. Practical Pain Management. 2009; 9(9):80-83.
Funding Source, Location of Study or Author’s Affiliation
Texas College of Osteopathic Medicine, Texas.
Device
Alpha-Stim® SCS
Key Variables
Pain
Objective
Investigate the effect of a specified treatment course with CES with pain. Consecutive treatments were performed to determine cumulative results.
Design
This study was an open label trial involving patients entering a pain clinic who were offered a complementary CES treatment. Patients that accepted the treatment were given a pain questionnaire and rated intensity on a 1–10 scale. The patients were asked to repeat this once the treatment was over. The patients also had the chance to come back for consecutive treatments for up to five days. This study was an open clinical trial.
Primary Outcome Measure
- The primary effectiveness endpoint was the change from baseline in the last post-treatment scores in pain.
- Change in pain level from baseline to post treatment.
Secondary Outcome Measure
None reported.
Key Inclusion Criteria
All patients who entered the pain clinic who did not meet the exclusion criteria.
Key Exclusion Criteria
- Pregnant
- Presence of implanted pacemaker, pump, or stimulator device.
Protocol Summary
Patients entering the Regional Pain Care Center of North Texas were offered the chance to receive a complementary CES treatment for their pain. Less than 1% of patients refused the treatment. Patients that participated filled out a pain questionnaire and rated their pain from 1–10 both before and after the CES treatment.
Device Application Protocol
Patients were able to adjust the current to a comfortable level and treated for 20 minutes. Most patients chose between 200 and 300 uA. Patients were also given the chance to come back for consecutive treatments for up to five days to test for cumulative improvement.
Statistical Analysis Plan
This was an open label study in which standard statistical methods were not used.
Results
Subjects
A sample of 525 consecutive patients who entered the Regional Pain Care Center of North Texas.
Data Analysis
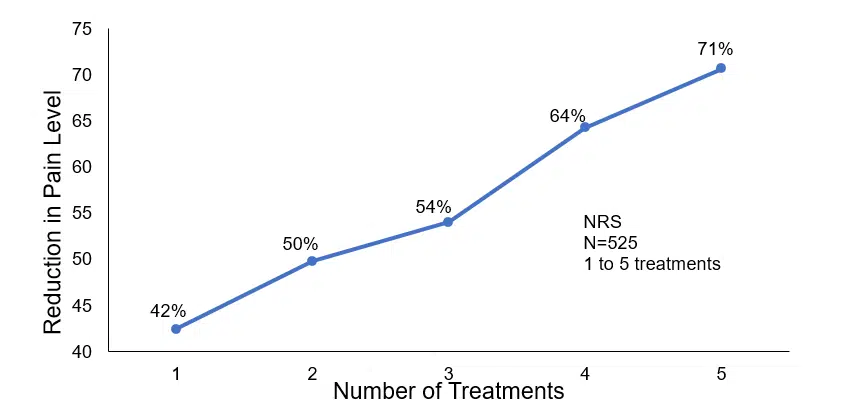
The percentage improvement in pain was calculated after each round of treatments. Fewer patients participated on the following day as more patients became pain free.
Cumulative improvement in pain after 1-5 CES treatments
Conclusion
One to five 20-minute CES treatment sessions produced a reduction in pain ranging from 42% to 71% in the approximately 80% of patients who responded. No negative side effects were observed by any member of the clinic staff or reported by the patients. Accordingly, this study gives credence to the claim that CES has a positive cumulative effect in refractory patients with a wide range of pain-related disorders.
Limitations
This study is not equivalent to a randomized controlled, double blind trial but it does provide important information regarding the role of CES in pain management. This study also illustrates the cumulative effect seen with continued use.
Study Quality: FAIR