Effect of CES Combined with Escitalopram on Quality of Life and Activities of Daily Living of Patients with Depression After Cerebral Infarction
Lyu, X. Effect of CES combined with escitalopram on quality of life and activities of daily living of patients with depression after cerebral infarction. Chinese Journal of Practical Nervous Diseases, 21(22), 2018.
Funding Source, Location of Study or Author’s Affiliation
The Tenth Ward, Luoyang Rongkang Hospital, Luoyang, China
Device
Alpha-Stim®
Key Variables
Post-Stroke Depression
Objective
To observe the effect of Cranial Electrotherapy Stimulation (CES) combined with escitalopram on quality of life and activities of daily living of patients with Depression after Cerebral Infarction or Post-Stroke Depression (PSD).
Design
Patients with a diagnosis of Acute Cerebral Infarction (ACI) and Post-Stroke Depression (PSD) were enrolled in a randomized sham controlled study of CES in addition to Treatment as Usual (TAU) inclusive of the antidepressant Escitalopram compared to a control group of TAU and Escitalopram only. Pre- and post-treatment scores were statistically analyzed to assess the efficacy of CES as an alternative or augmentation strategy for PSD, quality of life, and activities of daily living.
Primary Outcome Measure
- Depression as assessed using the Hamilton Depression Rating Scale (HAM-D)
- Neurological impairment as measured by the Modified Edinburgh-Scandinavia Stroke Scale (MESSS)
Secondary Outcome Measure
- Quality of life as measured by the World Health Organization Quality of Life Questionnaire abbreviated version (WHOQOL-BREF)
- Activities of daily living and independence as measured by the modified Barthel Index (BI)
Key Inclusion Criteria
- Acute Cerebral Infarction (ACI) confirmed via MRI examination and a Modified Edinburgh-Scandinavia Stroke Scale (MESSS) score of >16 points
- Post-Stroke Depression (PSD) as evidenced by a minimum HAM-D score of >18 points
Key Exclusion Criteria
- Prior history of depression or family depression
- Loss of consciousness (LOC)
- Limb dysfunction
- Congenital mental retardation
- Dysfunction of heart, liver, kidney, or other important organs, or otherwise unfit for CES
- Potential for poor compliance or unlikely to be able to adhere to study protocol
Protocol Summary
- The Control group was given Treatment as Usual (TAU) consistent with an ACI including medical observation and treatment (1. blood glucose and blood lipids management, 2. blood pressure monitoring and treatment as needed, 3. adequate water-salt balance, and 4. appropriate cerebral circulation). In addition, all patients received psychological counseling and pharmacological management with escitalopram. The initial dose was 5mg/day, then increased to 10mg/day after taking for five days, and the dosage was increased to 20mg/day as appropriate for four weeks continuously.
- The CES group received all of the same TAU management as the control group, inclusive of psychological and pharmacological treatment.
Device Application Protocol
The Alpha-Stim was used to administer CES. Patients were asked to lay supine and the electrodes were attached to the earlobes and stimulation frequency set to 0.5 Hz and current intensity to 10-500 μA. Each treatment session of CES was 60 minutes twice daily for the first two weeks, and once daily for the final two weeks.
Statistical Analysis Plan
SPSS 19.0 software was used for data analysis, and the change in WHOQOL-BREF, HAM-D, MESSS, and BI scores pre- and post-treatment were expressed by mean ± standard deviation and the comparison among groups was tested by t-test. A significance level of p<0.05 was used to infer a statistical significance.
Results
Subjects
A total of 160 patients were enrolled in the study, with 80 in each group. There were 46 males and 34 females in the control group aged between 45-70 years and with a mean age of 59.3±8.8 years. The CES group included 48 males and 32 females, aged between 46-70 years and with a mean age of 59.3±8.8 years.
Data Analysis
There were no significant differences at baseline in age or gender between the two groups (p>0.05).
At baseline quality of life scores between the two groups were not significantly different (p>0.05). At post-treatment, the CES group had significantly higher scores in quality of life over the control group (p<0.05).
| Group | n | Physical | Psychological | Social | Functional | Total average score of quality of life | |
| CES | 80 | Baseline | 20.2±3.4 | 16.1±4.7 | 14.4±2.1 | 22.3±4.8 | 17.9±4.2 |
| Post-treatment | 25.4±2.3*# | 20.9±3.2*# | 18.8±3.1*# | 25.8±3.3*# | 22.9±3.4*# | ||
| Control | 80 | Baseline | 19.7±3.6 | 15.8±4.8 | 14.2±3.1 | 22.1±4.4 | 17.6±4.1 |
| Post-treatment | 22.1±2.8* | 18.3±2.4* | 16.1±2.8* | 24.2±3.1* | 20.2±3.2 |
Note: Compared with that before treatment, *p<0.05; compared with control group, *p<0.05
WHOQOL-BREF scores between the 2 groups before and after treatment
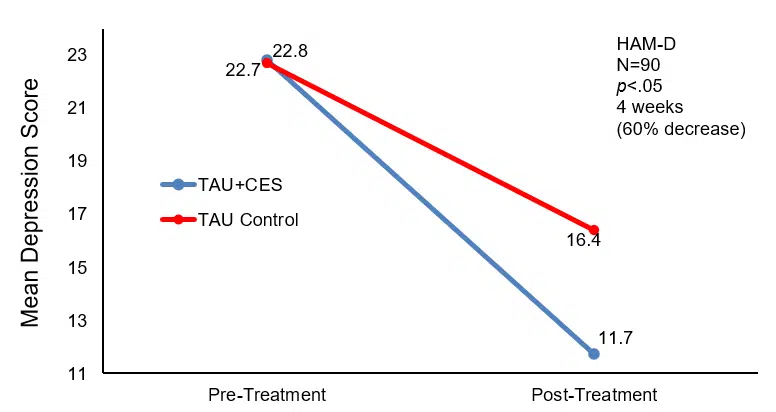
Before treatment, there was no significant difference in scores of HAM-D, MESS, or MBI between the two groups (p>0.05). After treatment, the scores of HAM-D and MESS of both groups decreased significantly (p<0.05), but the scores of the CES group were significantly lower than those of the control group (p<0.05). A reduction in scores is commensurate in a reduction in depression severity.
| Group | n | HAM-D | MESSS | BI | |
| CES | 80 | Baseline | 22.8±3.1 | 22.9±5.1 | 21.7±8.3 |
| Post-treatment | 11.7±4.8*# | 12.5±4.8*# | 68.3±8.5*# | ||
| Control | 80 | Baseline | 22.7±3.2 | 23.1±5.4 | 21.6±8.5 |
| Post-treatment | 16.4±5.7* | 17.1±5.4* | 57.2±3.9* |
Note: Compared with that before treatment, *p<0.05; compared with control group, #p<0.05
HAMD, MESS, and MBI scores between the 2 groups before and after treatment
Changes in Depression scores at baseline and post-treatment
Conclusion
A common complication of patients with cerebral infarction is depression. Referred to as Post-Stroke-Depression the commensurate behavioral and psychological changes negatively impact the rehabilitation process of patients. In addition, PSD is an important factor for recovery of neurological and motor deficits, and gradual improvement of activities of daily living. The authors explain that CES combined with escitalopram improves the quality of life of patients and enhances the treatment effect of escitalopram resulting in more effective and faster relief from depression symptoms. The authors conclude that improvements to patients’ depression promotes the recovery of neurological function resulting in improvements to activities of daily living and the quality of life of patients. CES is a safe and effective non-drug treatment for PSD and in conjunction with escitalopram improves the quality of life of patients and activities of daily living, and its effect is superior to the pure treatment of escitalopram.
Limitations
While this study was a randomized controlled trial, it was not double-blind. Therefore, both participants and investigators were aware of assigned intervention category. Additionally, the authors do not mention their procedures for missing data. According to the Cochrane risk-of-bias tool, each of these conditions can introduce unintended bias into a study.
Study Quality: FAIR