Effects of Cranial Electrotherapy Stimulation on Suicidal Ideation and Event-related Potentials in Patients with Treatment-resistant Depression
Gao, C., Kong, S., Duan, H., Yang, Z., Li, D. Effects of cranial electrotherapy stimulation on suicidal ideation and event-related potentials in patients with treatment-resistant depression. China Journal of Health Psychology. 2021; 29(2):183-188.
Funding Source, Location of Study, or Author’s Affiliation
Jiading District Mental Health Center, Shanghai, China
Mental Disease Prevention and Treatment Institute of Chinese People’s Liberation Army.
Device
Alpha-Stim® SCS
Key Variables
Suicide, Depression, and Physiological Markers.
Objective
To investigate the effect of Cranial Electrotherapy Stimulation (CES) on suicidal ideation and its electrophysiological mechanism in patients with depression.
Design
A total of 67 patients with Treatment-Resistant Depression (TRD) were randomly divided into two groups. The CES group received active CES treatment for eight weeks and the Control condition used an identical non-powered device. Before and after treatment suicidal ideation, depression, and physiological measures of patients in both groups were assessed.
Primary Outcome Measure
- Suicidal ideation
- as measured by the Beck Scale for Suicide (BSS) ideation – Chinese Version, (BSS-CV)
- including suicidal ideation and intent on 19 items and a severity level of 0-2 three-level on a maximum score of 38
- higher scores equating greater suicidal risk
- Depression severity
- as measured by the Hamilton Depression Rating Scale (HAM-D)
- a total of 17 items to evaluate the severity and a severity level of 0-4
- Score ranges: “Normal or no depression” ≤7, “Mild depression” 8-17; “Moderate depression” 18-24; “Severe depression” >24
Secondary Outcome Measure
Electrophysiological changes as measured by the Contingent Negative Variation (CNV).
Key Inclusion Criteria
- Diagnosis of Major Depressive Disorder as indicated by the International Classification for Disease (ICD-10)
- Meet criteria for Treatment-Resistant Depression (TRD) or prior history of 2 or more failed anti-depressant treatment trials at full-dosage and duration
- Hamilton Depression Rating (HAM-D) scale ≥18
- Aged 18-60 years
- No ECT or other physical therapy in the preceding four weeks.
Key Exclusion Criteria
- History of hypertension, diabetes or endocrine disease, other chronic diseases, or other serious physical diseases
- Prior history of other psychiatric disorder
- History of alcohol or substance abuse/dependency
Protocol Summary
Subjects were randomly divided into two groups using a random number table. The active CES and sham devices were identical, with the exception of the sham being a non-powered device. Treatment sessions were identical in duration and frequency. Pre and post assessment measures of suicide, depression, and electrophysiological measures were taken before and after treatment.
Device Application Protocol
CES treatment was given using the Alpha-Stim® SCS three times a week, 20 minutes every time for eight weeks. The earclips were attached to both earlobes with the clamp electrode, and a stimulation intensity between 10~500μA and frequency was set to 0.5Hz. Patients were able to gradually increase the intensity until they reported tingling or lightheadedness. The same connection method was also used in the control group, but the power of the device was off.
The CNV completed using an International Electroencephalogram Society approved brain electrophysiological instrument and in accordance with their recommendations. Effective electrode on head was put in Cz, the reference electrode was put in the right mastoid process and was connected with the ground electrode at 2cm above the midpoint of both eyebrows. The inductive stimulation mode consisted of S1 and S2, of which S1 was a short tone stimulation, and belonged to the prompt signal; S2 is Goggle flash stimulation and belonged to the command signal. Once the subject received S2, the key reaction was immediately done to interrupt S2. Analysis index: Incubation period and amplitude of M1 (early component) and M2 (late component), and occurrence rate of Post-Imperative Negative Variation (PINV).
Statistical Analysis Plan
Comparisons between the Active CES condition and the Sham Control on the BSS-CV, HAM-D, and CNV from baseline to post-treatment. SPSS 17.0 statistical package was used for data analysis, data were expressed as mean and standard deviation, and the independent sample and paired-sample were tested by t-test respectively; the enumeration data were tested by χ2 and a significance level set at p<.05.
Results
Subjects
A total of 67, with 33 in the CES group, including 18 males and 15 females with a mean age of 32.1±8.9 years, and mean years of education of 12.9±3.9 years. The control group included 34 subjects, including 18 males and 16 females, with mean age of 31.7±9.3 years, mean years of education of 12.6±3.7 years. The difference in gender, age, and years of education was not statistically significant.
Data Analysis
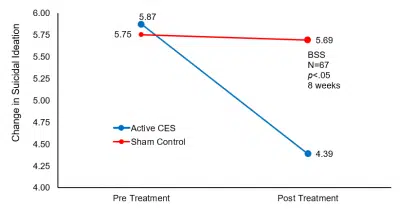
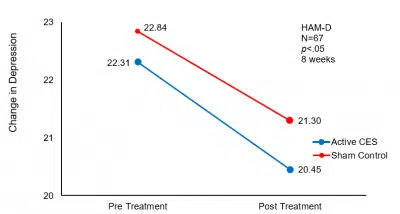
Baseline suicidal ideation, suicidal risk, and depression scores between the two groups were not statistically different, but after CES treatment, the scores of HAM-D (t=-2.0865, -2.1518; p<.05), and the scores of suicide ideation on the BSS (t= 2.0315, p<.05) in the CES group were significantly lower than that in the control group.
| Items | CES group (n=33) | Control group (n=34) | ||
| Before treatment | After treatment | Before treatment | After treatment | |
| Suicidal ideation | 5.87±3.21 | 4.39±2.51*△ | 5.75±3.11 | 5.69±2.72 |
| Suicidal risk | 11.98±6.85 | 9.53±5.36 | 11.67±6.54 | 11.78±6.97 |
| HAM-D | 22.31±3.22 | 20.45±3.78* | 22.84±3.62 | 21.30±3.14 |
Note: Through comparison with that before treatment, *p<.05; Through comparison with control group, △p<.05
Change of clinical symptoms before and after treatment
Mean change in suicidal ideation pre-and post- CES treatment.
Mean change in depression pre-and post- CES treatment.
Baseline CNV incubation period and amplitude, PINV occurrence rate, and other indexes between the two groups were not significantly different, but after treatment M2 amplitude increased and the incidence of PINV decreased in the CES group (t= 2.3513, p<.05; χ2= 5.0708, p<.05), representing a statistically significant difference compared to the control group (t =-2.0252. p<.05; χ2=4.7308, p<.05).
| Items | CES group (n=33) | Control group (n=34) | |||
| Before treatment | After treatment | Before treatment | After treatment | ||
| Incubation period (ms) | M1 | 452.3±143.5 | 424.8±138.9 | 443.7±151.9 | 451.8±142.6 |
| M2 | 1215.2±327.4 | 1168.8±309.7 | 1243.7±336.5 | 1195.4±317.5 | |
| Amplitude (μV) | M1 | 11.8±5.2 | 12.1±4.9 | 12.3±5.1 | 11.9±4.2 |
| M2 | 10.5±5.6 | 13.8±5.8*△ | 10.8±5.5 | 11.1±5.1 | |
| PINV occurrence rate% (n) | 39.4(13) | 12.1(4)*△ | 41.2(14) | 38.2(12) | |
Note: Through comparison with that before treatment, *p<.05; Through comparison with control group, △p<.05
Change of key indexes of CNV before and after treatment
Conclusion
The authors conclude that CES treatment can relieve the suicidal ideation of patients with major depressive disorder and treatment-resistant depression. The authors note CES treatment stimulates the production of certain neurotransmitters, such as 5-hydroxytryptamine, which also affects the chemical activity of a neuron’s peripheral region, further regulating activation. These electrophysiological changes may represent the mechanisms of action of CES at relieving suicidal ideation. The observable change in Event-Related Protocols (ERP) before and after CES treatment suggests that ERP may have clinical application as a potential warning or indicator of suicidal ideation that may encourage early diagnosis and appropriate treatment.
Limitations
While the design and results of this study are good, the recommended treatment frequency for Alpha-Stim CES is daily for the first three to six weeks, rather than three times a week, as was utilized in this study. Having participants treat daily for eight weeks may have resulted in more significant decreases in suicidal ideation and depression.
Study Quality: GOOD