Effects of Cranial Electrotherapy Stimulation on the Levels of HPA, BDNF and Clinical Symptoms in Patients with Mild Depression
Kong, S., Gao, C., & Yang, Z. Effects of Cranial Electrotherapy Stimulation on the Levels of HPA, BDNF and Clinical Symptoms in Patients with Mild Depression. Journal of International Psychiatry. 48(1), 2021.
Funding Source, Location of Study or Author’s Affiliation
Jiading District Mental Health Center, Shanghai
Device
Alpha-Stim® SCS
Key Variables
Anxiety, Insomnia, and Depression.
Objective
To investigate the effects of cranial electrotherapy stimulation (CES) on the hypothalamic-pituitary-adrenal (HPA) axis, levels of brain-derived neurotrophic factor (BDNF), and clinical symptoms in patients with mild depression.
Design
A randomized sham-controlled study of eighty-six patients with mild depression divided into two groups. Blood samples were collected before and after 8 weeks of treatment to measure cortisol (COR), adrenocorticotropic hormone (ACTH), and brain-derived neurotrophic factor (BDNF). Accompanying depression, anxiety, and sleep measures were taken pre and post-treatment. Depression was measured using the Hamilton Depression (HAM-D) and anxiety using the Hamilton Anxiety (HAM-A) rating scales. Insomnia was assessed using the Insomnia Severity Index (ISI). Patients received either active or sham CES for 20 minutes per session, 3 times per week, over 8 weeks.
Primary Outcome Measure
Blood serum levels of
- COR
- ACTH
- BDNF
Secondary Outcome Measure
- Depression, as evaluated using the 17 items HAM-D rating scale. A total HAM-D total score of (i) 0–7 is considered “Normal”, (ii) 8–16 “Mild depression”, (iii) 17–23 “Moderate depression”, (iv) > 24 indicative of “Severe depression.”
- Anxiety, as evaluated using the 14 item HAM-A rating scale. A HAM-A total score of (i) < 7 indicates “No anxiety”, (ii) 7-13 “Possible anxiety”, (iii) 14-20 “Definite anxiety”, (iv) 21-28 “Definitive and obvious anxiety”, and (v) ≥ 29 “Possibly severe anxiety”.
- Sleep as measured by the ISI and indicative of the quality of sleep in the previous two weeks. The ISI includes 7 items scored over 5 levels (0-4) with severity from mild to severe. A total score (i) <7 indicates “Absent of insomnia”, (ii) 8-14, “Mild insomnia”, (iii) 15-21 “Moderate insomnia”, and (iv) 22-28, “Severe insomnia”.
Key Inclusion Criteria
- Depression – meet diagnostic criteria as per the International Classification of Diseases (ICD-10) for mild depression or a HAM-D score of 17-24.
- Anxiety – a HAM-A score of 7-20.
- Age – between 18-60 years.
- Body Mass Index (BMI) lower than 30kg/m2.
- No antidepressant medication, electric convulsive treatment (ECT), or other psychiatric treatments in the past four weeks.
Key Exclusion Criteria
Psychiatric History
- History of other psychiatric disorders.
- Active or prior history of suicidal ideation or intent or the emerge of during treatment.
- History of alcohol or substance abuse/dependence
Chronic Conditions – diagnosis of hypertension, diabetes, endocrine disease, other chronic diseases, or serious physical diseases.
Protocol Summary
A total of 86 patients with mild depression were enrolled in the study. Subjects were randomly assigned to the study group and control group using a random number table. All subjects were diagnosed with mild depression and underwent a weekly psychological evaluation and were withdrawn from the study if their symptoms worsened or they developed suicidal ideation or intent.
Weight
Subjects’ height and weight were measured using a DST-500 ultrasonic wave height and weight measuring apparatus and calculated as BMI=Weight/height 2 (kg/cm2).
Blood Sampling
A sample of 8ml of venous blood was taken at the elbow following a period of fasting into anticoagulant-free tubes for serum preparation and 1.5ml of blood was collected in tubes for plasma and supernatant preparation and placed in a thermostatic water bath for 1h. Blood samples were centrifuged at 3,000r/min for 5min, then centrifuged plasma was stored at -80℃ in a refrigerator until levels of COR, ACTH and BDNF were assayed. Samples were taken before and after 8 weeks of treatment and used to assess change in COR, ACTH, and BDNF levels.
Hormone Assessment
COR was tested using the Roche E170 kit and was analyzed by electrochemiluminescence.
ACTH was tested using the France CIS Bio International kit and was determined by enzyme-linked immunosorbent assay (ELISA).
BDNF determination:
BDNF level was centrally determined by ELISA, the coefficient of inter-batch variation was less than 5%, and the coefficient of intra-batch variation was less than 4%. The kit was provided by Wuhan Boster Biological Technology Co., Ltd., sensitivity: <2pg/mL, scope: 31.2-2,000pg/mL.
Device Application Protocol
Patients in the active and sham conditions received CES treatment using the Alpha-Stim® SCS device. The treatment protocol was 3 times every week, for 20 minutes, over 8 weeks. The clamp electrodes in the CES device were attached bilaterally to the earlobes and the electrodes connected to the device. The intensity was set to 10-500 μA and the frequency to 0.5Hz. Patients were able to gradually increase the intensity until they felt a mild tingling sensation. The device used in the sham condition was applied in the same way, but the power of the device was turned off.
Statistical Analysis Plan
The data were analyzed using SPSS 17.0 statistical package. The measurement data showed the mean and standard deviation, and the independent sample and paired-sample were tested by t-test; The enumeration data were tested by χ2 and a significance level set to p<.05.
Results
Subjects
A total of 86 patients with mild depression were enrolled in the study. No significant differences between the active and sham groups in terms of gender (X2=0.074, p=.8276), age (t=0.2181, p=.8278), education level (t=-0.5362, p=.5932), and BMI (t=-0.4880,p=.6268) were reported.
- In the active CES group, 43 patients – 25 males and 18 females, aged between 18-52 years old, mean age of 31.5±8.3years were enrolled. The mean education level of the active CES group was 12.8±3.7 with a range of 9-16 years, and a mean BMI of 23.8±2.8kg/m2.
- In the sham CES condition, a total of 43 patients were enrolled, 24 males and 19 females aged between 18-54 years, with a mean age of 31.9±8.7 years. The education level for the sham CES group ranged between 9-16 years, with a mean of 12.4±3.2 years, and a mean BMI of 24.1±2.9kg/m2.
Data Analysis
Anxiety, Depression, and Insomnia
The difference between HAM-D, HAM-A, and ISI of the active and sham CES condition before treatment was not statistically significant (t=-1.801, -0.8956, 0.6223; p=.2413, 0.3730, 0.5354).
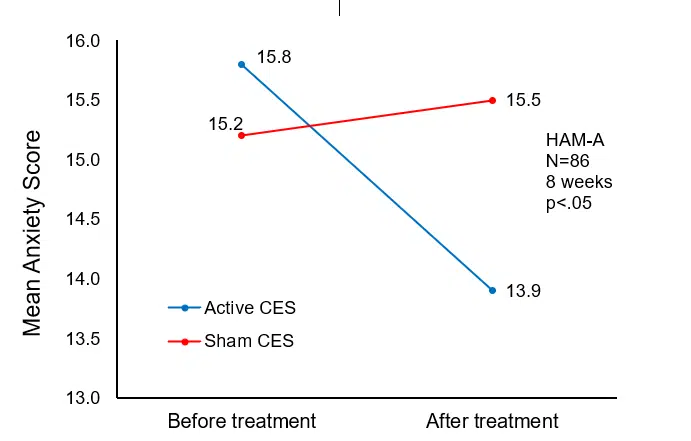
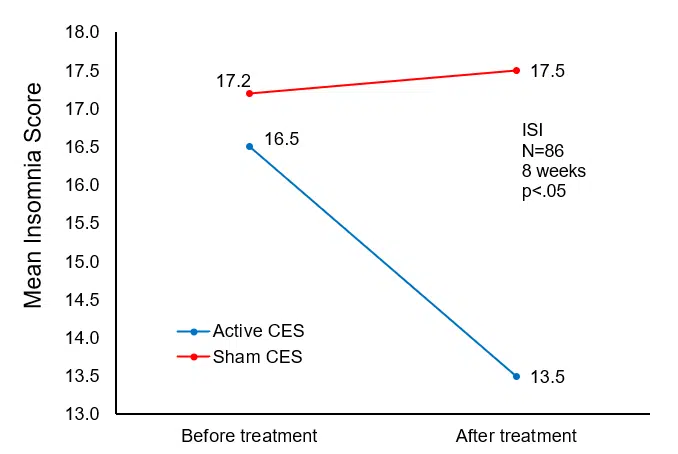
After treatment, the HAM-A and ISI scores in the active CES group decreased significantly (t=-2.5512, -2.7228; p=.0125, 0.0079) and were significantly lower than the sham CES group (t=2.1188, 3.2795; p=.0371, 0.0015).
The change in HAM-D from baseline to post-treatment was significant (t=-1.7267; p=.0879). However, the difference between the active and sham conditions was not statistically significant (t=-1.6207; p=.1088).
The change in HAM-D, HAM-A, and ISI before and after treatment in the sham group was not statistically significant (t=-1.0497, 0.4402, 0.2418; p=.2969, 0.6609, 0.8095).
Change in clinical symptoms of anxiety, depression, and sleep in the active and sham CES group before and after treatment
| Items | Active CES (n=43) | Sham CES (n=43) | ||
| Before treatment | After treatment | Before treatment | After treatment | |
| HAM-D | 20.5±2.8 | 19.4±3.1 | 19.8±2.7 | 20.4±2.6 |
| HAM-A | 15.8±3.3 | 13.9±3.6*△ | 15.2±2.9 | 15.5±3.4 |
| ISI | 16.5±4.8 | 13.5±5.4**△△ | 17.2±5.6 | 17.5±5.9 |
Note: HAM-D (Hamilton Depression Scale); HAM-A (Hamilton Anxiety Scale); ISI (Insomnia Severity Index).
Compared with baseline, **p<.01, *p<.05; Compared with the sham CES condition, △△p<.01, △p<.05.
Change in mean anxiety (HAM-A) scores from pre- to post-treatment.
Change in mean insomnia (ISI) scores from pre- to post-treatment
HPA and BDNF levels
The were no significant differences in baseline levels of COR, ACTH, and BDNF between the active and sham CES groups (t=0.3088, -0.4415, 0.4717; p=.7582, 0.6600, 0.6383).
After treatment, the concentration of COR in the active CES group decreased (t=-2.0538; p=.0431) and was significantly lower than that of the sham condition (t=2.3613; p=.0205).
The change in ACTH and BDNF levels from baseline to post-treatment was not significant (t=0.2788, 0.9442; p=.7811, 0.3478), and the difference between the active and sham conditions was also not statistically significant (t=-1.0609,-0.7806; p=.2918, 0.4372).
The change in ACTH and BDNF before and after treatment in the sham CES condition was not statistically significant (t=-0.2102, -0.2425, -0.3333; p=.8340, 0.8090, 0.7398).
Change in HPA axis regulating hormone and BDNF in the active and sham CES group before and after treatment
| Items | Active CES (n=43) | Sham CES (n=43) | |||
| Before treatment | After treatment | Before treatment | After treatment | ||
| COR (nmol/d) | 435.3±147.7 | 372.5±135.6*△ | 446.1±175.4 | 454.2±181.9 | |
| ACTH (pg/m) | 45.6±28.9 | 47.2±24.1 | 42.9±27.8 | 41.5±25.7 | |
| BDNF (pg/m) | 28.1±11.5 | 31.2±18.2 | 29.5±15.7 | 28.4±14.9 | |
Note: COR (cortisol); ACTH (adrenocorticotropic hormone); BDNF (brain-derived neurotrophic factor).
Compared to baseline, *p<.05; Compared with the sham CES condition, △p<.05.
After CES treatment, the level of COR, and HAM- A and ISI scores in the active CES group decreased significantly (t=-2.0538, – 2.5512, – 2.7228; p=.0431, 0.0125, 0.0079), and was significantly lower than the sham CES group (t= 2.3613, 2.1188, 3.2795; p=.0205, 0.0371, 0.0015).
HAM-D and ACTH did not show a significant change from baseline to post-treatment (p>0.05) in either the active or the sham CES conditions.
Conclusion
The results from the study demonstrated CES treatment resulted in changes to COR plasma concentrations in patients with mild depression but did not cause significant changes to levels of ACTH and BDNF. The authors concluded that CES treatment plays a positive role in improving anxiety and insomnia symptoms in patients with mild depression, but its effect on depression was poor. Stating that the observable changes in hormone levels of patients with mild depression following CES treatment are likely associated with stimulation of the HPA axis, and the reason for the accompanying reduction in symptoms of anxiety and insomnia, but not depression. The authors proposed that stimulation of the HPA axis is likely one of the possible mechanisms of action CES.
Limitations
The choice of treatment protocol used in this study is inconsistent with the Instructions For Use in treating depression with Alpha-Stim CES. The Owner’s Manual indicates daily treatment for a minimum of three weeks is necessary to treat depression. The protocol of treating three times a week may not have been sufficient to allow participants to receive the full benefit of Alpha-Stim treatment for depression. This study also did not follow the EPI protocol for a double-blind randomized controlled trial, which is to set the active devices to 100 uA for 60 minutes and for the sham devices to be set identically to the active devices, but not emit a current.
Study Quality: FAIR
 100vw, 703px” data-lazy-src=”https://alpha-stim.com/wp-content/uploads/2021/08/Kong-depression.png” /></a></p><p><strong>Change in mean depression (HAM-D) scores from pre- to post-treatment.</strong></p><p><a href=)