Efficacy of Cranial Electric Stimulation for the Treatment of Insomnia: A randomized pilot study
Lande, R. Gregory and Gragnani, Cynthia. Efficacy of cranial electric stimulation for the treatment of insomnia: A randomized pilot study. Complementary Therapies in Medicine, 21(1):8-13, 2013.
Device
Alpha-Stim®
Key Variable
Insomnia
Objective
The purpose of this pilot study was to examine the potential efficacy of CES for the treatment of insomnia.
Design
This was an IRB approved 5 day pilot study that used a randomized, sham controlled, double-blind design.
Primary Effectiveness Endpoint
The primary effectiveness endpoint was the change from baseline in the total sleep time (from sleep log) for the active group compared to the sham treatment group at the endpoint of study.
Secondary Outcome Measures
The secondary outcome measure was the change from baseline in the last post-treatment scores on the time to sleep onset and number of awakenings from sleep logs for active CES subjects compared to the sham treatment at the endpoint of study.
Key Inclusion Criteria
• Male and female active duty Service Members receiving mental health care
at the Psychiatry Continuity Service at Walter Reed National Military Medical Center, Bethesda, Maryland.
• Must have score ≥ 21 on the psychiatric impairment rating scale (PIRS).
Key Exclusion Criteria
• Pregnancy, planning to become pregnant or nursing.
• Presence of implanted cardiac pacemakers, pumps or electrical stimulators.
• Subjects determined by clinical evaluation and self-administered psychometric tests as actively suicidal, having a seizure disorder history or active vertigo.
Protocol Summary
The device manufacturer pre-set and locked the active and sham devices at the specified settings. The active CES device was set and locked at 100 µA. The sham CES device was set and locked at “0” so that it did not emit electricity. Subjects were randomly assigned to a sham or active CES group by the investigator who randomly selected a device from the box containing 10 active CES devices and 10 sham CES devices. Each subject received a 60 minute active or sham CES treatment daily for 5 days. Subjects completed a sleep log daily for the 5 days. After the 5 days, subjects completed a sleep log at 2 follow-up points, 3 days and 10 days.
Device Application Protocol
The active CES device was pre-set and locked by the manufacturer at 100 µA which is a subsensory level. The sham CES device was pre-set and locked by the manufacturer so that it did not emit electricity. The sham device was identical to the active CES device, except it did not emit electricity. The 60 minutes length of treatment was also pre-set and locked by the manufacturer for both active and sham devices.
Study Blinding
The subjects, investigators and staff were all masked as to the identity of the device, active or sham.
Outcome Measures
The total sleep time was measured from subjects’ reports in their daily sleep log.
Results
Subjects
Fifty-seven (57) subjects enrolled and completed the study; 46 males and 11 females. There were 28 in the active CES group and 29 in the sham CES group. Over three-quarters of the subjects completed the full 5 CES treatments (N=44, 77%).
Data Analysis
Data were analyzed using descriptive statistics, chi-square, 2 way analysis of variance and independent sample t-tests.
Total Sleep Time
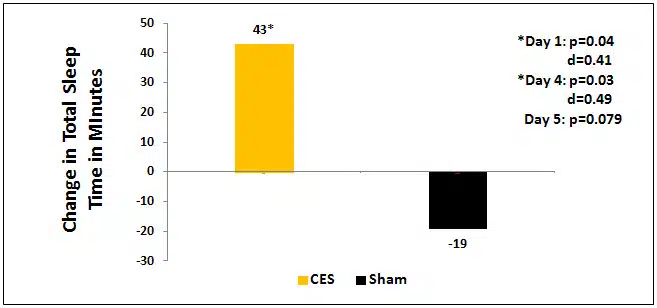
The total time slept approached significance (p=0.079) on day 5 in favor of the active CES group. The active CES group average about 43 extra minutes total sleep time when compared to the sham CES group subjects who reported an average of 19 minutes less sleep time. A gender difference also emerged. Men in the active CES group who completed 5 sessions of CES reported a significant improvement in total time slept at 2 points in the study, after the initial (p=0.04, d=0.41) and after the fourth (p=0.03, d=0.49) treatments as compared to men in the sham group. There were no significant changes among the females.
Figure 1. Change in total sleep time in minutes between the active group, which had plus 43 more minutes of sleep and the sham group which had 19 fewer minutes of sleep a night after only 5 treatment sessions (p=0.079).
Quality of the Research
This pilot study was done in preparation for a grant proposal submission. Strengths of the study are: the use of a randomized, double-blind, sham controlled design; and the detailed CES device protocol and the structured protocol for the CES treatments. The major limitation of this study is the number of CES treatments. For the treatment of insomnia the recommended protocol is a minimum of daily CES treatments for at least 4 weeks and 6 to 8 weeks is sometimes required. The investigators state that the small N of the study was a limitation of the study. However, based on the effect size for insomnia, the N of 57 is adequate to detect the effect of CES if subjects in the active CES group have the recommended amount of daily treatments, 4 to 8 weeks.
Author Affiliations
R. Gregory Lande. Psychiatric Continuity Service, Walter Reed National Military Medical Center, Bethesda, MD. Cynthia Gragnani, Psychiatric Continuity Service, Walter Reed National Military Medical Center, Bethesda, MD