Prospective Study of Brain Wave Changes Associated with Cranial Electrotherapy Stimulation
Lande GR and Gragnani CT. Prospective study of brain wave changes associated with cranial electrotherapy stimulation. Primary Care Companion for CNS Disorders, 2018; 20(1).
Funding Source, Location of Study or Author’s Affiliation
This study was conducted at Psychiatric Continuity Services at Walter Reed National Military Medical Center, Bethesda, Maryland.
Device
Alpha-Stim® AID
Key Variables
Electroencephalogram (EEG) and distress.
Objective
The objective of this study was to explore brain wave changes associated with cranial electrotherapy stimulation (CES) among subjects receiving psychiatric care.
Design
This was an open label, prospective, convenience sample study.
Primary Outcome Measure
The primary effectiveness endpoint was qEEG changes when comparing qEEG results pre- and post-CES treatment.
Secondary Outcome Measure
Subjective Units of Distress Scale.
Key Inclusion Criteria
For purposes of screening clinical conditions, the following thresholds were applied:
- Alcohol Use Disorders Identification Test (AUDIT) ≥8
- PTSD Checklist for DSM-5 (PCL-5) ≥33
- Pittsburgh Insomnia Rating Scale (PIRS) ≥21
- Zung Self-Rating Depression Scale (SDS) ≥45
- Zung Self-Rating Anxiety Scale (SAS) ≥45
Key Exclusion Criteria
None reported.
Protocol Summary
Subjects supplied qEEG data was acquired from a wireless single channel EEG of CES treatment at a comfortable level. Thirty second measurements of brainwave activity were measured at baseline immediately preceding CES, immediately after the 20-minute CES treatment, and 10 minutes after CES.
Device Application Protocol
An investigator assured proper positioning of the CES earclips and then adjusted the microamperage to the point of mild subjective discomfort, then reduced the microamperage to eliminate the sensation, which replicates the process performed in clinical practice. Subjects received CES for 20 minutes.
Statistical Analysis Plan
Apriori hypothesis was that application of an Alpha-Stim would cause changes in the EEG.
Results
Subjects
The study was conducted among 50 active-duty service members receiving treatment at the Psychiatry Continuity Service, Walter Reed National Military Medical Center in Bethesda, Maryland. The Psychiatry Continuity Service is an outpatient partial hospital program staffed by a group of multidisciplinary clinicians and provides intensive care for all psychiatric disorders requiring this level of treatment. The typical subject was mildly depressed and had severe trauma-related symptoms and sleep problems.
Data Analysis
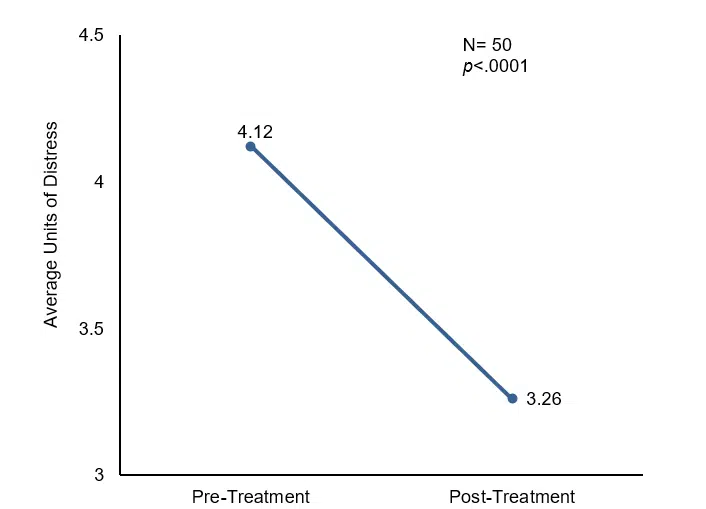
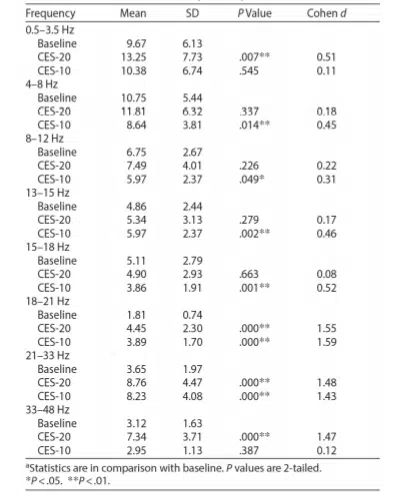
There was a significant increase (p=.000) in the higher beta frequencies (18-21 Hz, 21-33 Hz, and 33-48 Hz) and a strong effect (with the Cohen d around 1.5) immediately following the 20-minute CES. Ten minutes after CES, slower wave activity (4-8 Hz and 8-12 Hz) significantly decreased (p<.05), while increases in beta wave activity persisted (13-15 Hz, 18-21 Hz, and 21-33 Hz). A strong effect (with the Cohen d around 1.5) persisted in the beta brain wave bands 18-21 Hz and 21-33 Hz. There was also a significant difference (p=.000) in the subjective units of distress scale before CES (mean=4.12) and after CES (mean=3.26).
Change in units of distress pre- and post-treatment.
Changes in brain wave activity after 20 minutes of CES (CES-20) and 10 minutes later (CES-10).
Conclusion
Brain wave measurements taken immediately after the 20-minute CES session showed a significant and strong effect in the beta region, suggesting an increase in mental alertness, focus and concentration. Significant changes occurred as quickly as 10 minutes and the strong effect in the beta region persisted through the 10-minute follow up, indicating increased mental alertness. Participants also reported significant reduction in distress following the CES treatment. This finding may be related to the increase in beta wave activity. Improved mental focus and corresponding decrease in distraction may be a welcome relief among individuals with overlapping anxiety, depression and trauma symptoms as reflected in this study group.
Limitations
The authors state this open-label study attempted to balance clinical use of Alpha-Stim with scientific rigor. The subjects were exposed to limited interactions, such as positioning the brain wave-sensing headset, with the investigators. While other factors such as the subject’s awareness of the stimulation phase of CES may impose a potential bias on the study’s results, it is at least partially mitigated by the number of subjects, the strength of the findings, and the value in determining the role of microamperage dosing.
Study Quality: FAIR