Sertraline combined Alpha-Stim clinical observations on the treatment of 30 cases of generalized anxiety disorder
Ou, Y. & Li, C. Sertraline combined Alpha-Stim clinical observations on the treatment of 30 cases of generalized anxiety disorder. Chinese Journal of Ethnomedicine and Ethnopharmacy. 2015; 24(17):73-75.
Funding Source, Location of Study or Author’s Affiliation
The Second People’s Hospital of Pingxiang City Jiangxi Province, Pingxiang, China
Device
Alpha-Stim®
Key Variables
Anxiety
Objective
Evaluation of sertraline combined with Alpha-Stim on clinical efficacy and safety in the treatment of generalized anxiety disorder (GAD).
Design
A group of sixty patients diagnosed with GAD were randomly allocated into a control group (MED) that received treatment-as-usual (TAU) consisting of Sertraline. The experimental group received 6 weeks of cranial electrotherapy stimulation (CES) in addition to medication (MED+CES). Baseline and post-treatment scores in the Hamilton Anxiety Rating Scale (HAM-A) were used to assess clinical response. The side effect profile of each treatment was measured using the Treatment Emergent Symptom Scale (TESS).
Primary Outcome Measure
- Change in GAD symptoms as measured by the HAM-A before and after treatment. A HAM-A total severity score of (i) < 7 indicates “No anxiety”; (ii) 7-13 “Possible anxiety”; (iii) 14-20 “Definite anxiety”; (iv) 21-28 “Definite and obvious anxiety”; and (v) ≥ 29 “Possibly severe anxiety”.
Secondary Outcome Measure
- Prevalence of side effects as measured by the TESS. The TESS is used to assess adverse events that first occurred or worsened in severity after the initiation of therapy. The scale includes measures of behavioral toxicity, laboratory abnormalities, cardiovascular, autonomic, and nervous system reactions, body weight, headache, and appetite changes. Scores range from 0 to 4, with a higher score indicative of more serious adverse reactions.
Key Inclusion Criteria
- Meet ICD-10 diagnostic criteria for GAD.
- Normal blood and urine analysis, liver and renal function assessment, and electrocardiogram (ECG).
Key Exclusion Criteria
- Organic brain disease.
- Alcohol, drug, or substance abuse or dependence.
- Severe physical disease.
- Pregnant or lactating.
- Medical history contraindicating the use of Alpha-Stim CES
- History of epilepsy.
- An implanted electronic device such as a cardiac pacemaker or insulin pump.
- History of symptoms associated with a hemorrhagic stroke.
- Monoamine oxidase (MAOIs) use within two weeks prior to study start.
Protocol Summary
A group of 60 patients diagnosed with GAD were randomly allocated into one of two groups. Treatment efficacy involved comparing pre and post-anxiety severity using the HAM-A. In addition, any side effects of treatment were measured using the TESS.
The control condition received treatment-as-usual (TAU) medication. Patients in the MED group were prescribed an initial dose of 50mg/d of Sertraline and up to a maximum dose of 200mg/d depending on anxiety severity and the patients’ tolerance. The mean Sertraline dosage of the MED group was 73.5±23.7mg/d. The MED+CES group received six weeks of CES in addition to TAU with Sertraline. The Sertraline dosage in the MED+CES group was identical to the MED group with a mean of 65.5±15.6mg/d.
Device Application Protocol
Alpha-Stim was used in the MED+CES group to administer daily CES treatment for six weeks, each treatment session was 20 minutes. Earclip electrodes were clamped to the patient’s earlobes and the stimulation intensity was set to between 10-500 μA, the frequency was set to 0.5 Hz, with an output pulse width of 0.5s.
Statistical Analysis Plan
Clinical efficacy was observed by comparing the change in HAM-A score. A patient whose HAM-A score from baseline to post-treatment was reduced by ≥75% was labeled as healed; 50%-74% as making significant progress; 25%-49% as having improved; and <24% as remaining unchanged.
SPSS 16.0 software was used for data processing, the enumeration data were tested by χ2, and the measurement data by t test. A p<.05 level was set as indicative of a statistically significant difference.
Results
Subjects
A total of 60 subjects were enrolled in the study, 36 females and 24 males, aged between 20 to 61 years. The MED+CES group included 30 patients, 13 males, and 17 females, mean age of 47.1±9.6 years. The MED group included 30 patients, 11 males, and 19 females, mean age of 46.3±9.3 years. Statistical analysis at baseline showed no significant differences between the groups in terms of gender and age, and the groups were considered comparable (p>.05).
Data Analysis
From the first week of treatment, the reduction in total HAM-A score from baseline in the MED+CES group compared to the MED group was significantly lower (p<.05). In addition, the Sertraline dosage of patients in the MED+CES group reporting a reduction in anxiety levels is lower than the patients in the MED group. The lower dosage of medication in the MED+CES group equated to a total lower incidence of adverse reactions (p<.05).
Anxiety
The baseline HAM-A scores were 24.12±7.58 and after the first week of treatment, they were reduced to 17.36±5.76, a statistically significant difference (p<.05). The significant reduction in HAM-A scores in the MED+CES group shows that Sertraline combined with CES for the treatment of GAD results in rapid treatment response. The differences in HAM-A scores were also significantly lower (p<.01) at the end of weeks two and four. However, at the end of the sixth week, the comparative difference in HAM-A scores between the two groups wasn’t statistically significant (p>.05), as shown in the table and graph below.
| Measurement time | MED + CES (n=30) | MED (n=30) |
| Baseline | 24.12±7.58 | 23.85±7.82 |
| The 1st week of treatment | 17.36±5.76* | 20.95±7.61 |
| The 2nd week of treatment | 10.72±5.82** | 17.58±6.22 |
| The 4th week of treatment | 6.23±4.56** | 10.58±4.36 |
| The 6th week of treatment | 5.26±3.64 | 6.98±4.52 |
Note: Through comparison with the control (MED) group, *p<.05, **p<.01
Comparison of HAM-A scores at different times of treatment
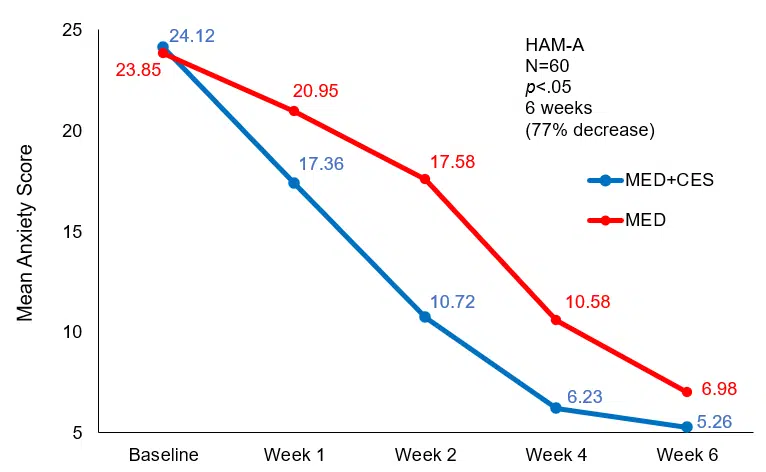
Mean anxiety (HAM-A) score from baseline to end of treatment for the control (MED) and experimental (MED+CES) group.
Efficacy
The comparative difference in clinical efficacy between the two treatment groups was not statistically significant (p>.05). However, as the table below indicates, the total effective rate percentage for the MED+CES group is greater than the MED group.
| Group | Case | Healed | Significant Progress | Improved | Unchanged | Total effective rate % |
| MED+CES | 30 | 15 | 10 | 3 | 2 | 93.5 |
| MED | 30 | 9 | 8 | 5 | 8 | 73.3 |
Comparison of clinical efficacy
Safety
The comparative difference in the occurrence of adverse reactions as measured by the TESS during the six weeks of treatment in the two groups wasn’t statistically significant (p<0.05). However, the total occurrence of adverse events was significantly higher in the MED than the MED+CES group, as shown below.
| Adverse reactions | MED+CES | MED |
| Nausea | 1 | 3 |
| Loss of appetite | 0 | 3 |
| Headache | 1 | 0 |
| Dizziness | 0 | 1 |
| Loss of appetite | 2 | 0 |
| Thirst | 0 | 3 |
| Transaminase rise | 0 | 2 |
| Palpitation | 0 | 1 |
| Total occurrence rate/% | 13.3* | 43.3 |
Note: Compared with the control (MED) group, *p<.05
Frequency of adverse events (TESS)
Conclusion
Sertraline combined with Alpha-Stim for the treatment of GAD is fast, safe, and effective. The results from this study show that medication plus CES leads to rapid treatment response, reduced medication dose, low side effect profile, and greater patient treatment compliance. Consequently, the authors concluded that CES deserves to be clinically promoted and applied, particularly as an augmentation strategy to traditional medication treatment for GAD.
Limitations
While the results of this study are good, the CES protocol utilized is not consistent with other studies or with clinical instructions for use. For participants who were able to tolerate currents higher than 250 uA, the 20-minute treatment duration is sufficient, but for patients requiring lower than 200 uA, 60 minutes of treatment is recommended. Consequently, the efficacy of CES compared to medication only or as the role of CES as an augmentation strategy is likely to be even higher than reported in the current study. Additionally, while the randomization technique in this study is effective, neither the investigators nor the participants were blinded to the treatment condition, which could introduce unintentional bias into the results, according to the Cochrane Risk of Bias Tool.
Study Quality: FAIR
